TrialAssure Uses Artificial Intelligence to Create Plain Language Summaries for 50,000 Studies Listed on ClinicalTrials.Gov
- TrialAssure’s proprietary AI-technology can aid in making clinical trial results available more quickly to all trial participants and the public
- The equivalent of one million work hours completed in less than one day using AI in honor of Health Literacy Month
- Plain language summaries to be released over a five-week period by disease or condition on TrialResults.com
- All clinical trials that have posted results on ClinicalTrials.Gov since 2020 have been included in what may be the single largest patient engagement effort using AI in pharma
DETROIT (October 18, 2023) – TrialAssure, an AI-technology leader in advancing clinical trial results disclosure and data sharing in the pharmaceutical industry, announced today that it has taken the proactive effort to create and publish clinical trial results in plain language summary (PLS) format, posting directly to TrialResults.com. This includes plain language summaries for every clinical trial that has had results posted on the National Institutes of Health’s (NIH) ClinicalTrials.gov website since 2020 – nearly 50,000 documents.
The documents were created using clinical data provided by the clinical trial sponsors to the federal registry (ClinicalTrials.gov) and using generative artificial intelligence (AI) that is built into TrialAssure LINK® – a proprietary technology that efficiently develops, translates, and publishes plain language summaries to meet increasing compliance requirements and the growing public demand for non-technical clinical trial information.
Plain language summaries, a regulatory requirement elsewhere in the world, are meant to provide the public with the results of a clinical trial in a way that is more easily understood (i.e., 6th-8th grade reading level). While still voluntary in the United States, there has been increased public outcry for transparency in the pharmaceutical industry and these plain language summary documents are required in the European Union (EU) under the EU Clinical Trial Regulation No. 536/2014.
This comes at a time when just 12 percent of Americans are considered proficient in their health literacy, according to the National Assessment of Adult Literacy, and most recent research out of the NIH shows that only about 14 percent of plain language summaries are available to clinical trial participants.
How does this help patient engagement?
“These health literacy numbers need to improve, and one way forward is to share information in formats that are accessible. AI-led technology like TrialAssure LINK will help the medical community, including researchers in biopharma, to more clearly communicate with participants and their families as to the results of the clinical trials and also increase treatment options. This initiative recognizes the importance of our collaboration, especially during Health Literacy Month this October,” said Prasad M. Koppolu, Chief Operating Officer, TrialAssure.
Koppolu continued, “By using TrialResults.com, we have charged past the lack of a current mandate for reporting these plain language summary documents and taken a step forward, posting understandable clinical trial results to help improve health literacy and give the entire pharmaceutical industry an opportunity to be more transparent.”
Plain language summaries help patients, their families, and the public at large by:
- providing a better understanding of the clinical study that occurred,
- giving more context and details around what the individual studies accomplished, or didn’t accomplish, and
- helping them understand the value of personal contributions to science and empowering clinical trial participants to continue to be involved in clinical trials in the future.
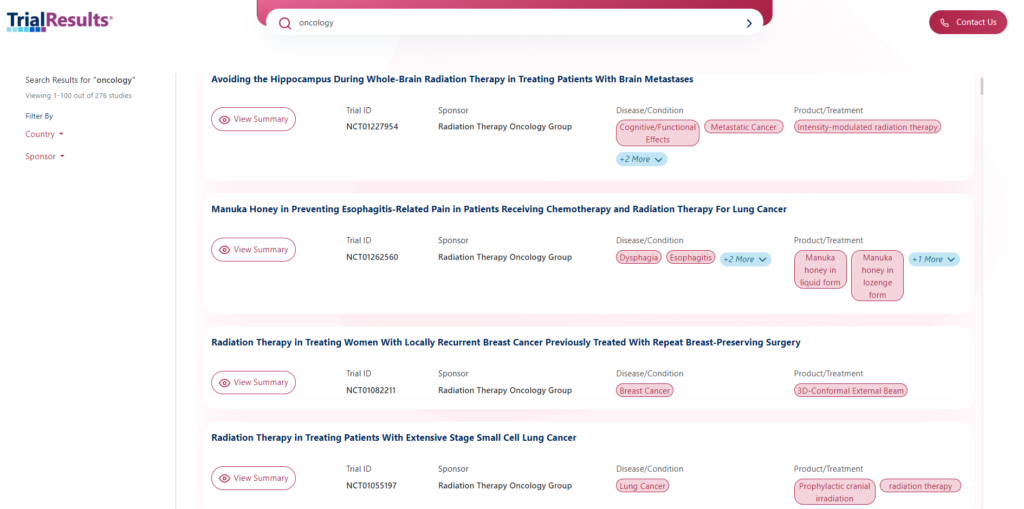
On the TrialResults.com website, the public can learn about specific clinical trials for diseases or conditions of interest. Reading one of the approximately 50,000 plain language summaries available provides a starting point to understand the clinical trial at a high level – just enter a disease, condition, or study number in the search bar. The study sponsor will always be listed in the summary, making it easy for individuals to find direct and additional information on the sponsor website.
The plain language summary documents will be released on TrialResults.com in the following five groupings by condition and/or disease:
- Oncology (available now)
- Metabolic
- Mental Health and Neurological
- Respiratory
- All others
To be notified when additional summaries are posted, email connect@trialassure.com to be added to the mailing list.
Reviewing the ClinicalTrials.gov record for further supporting data and understanding is recommended and a link will be available on TrialResults.com for each record. It should be emphasized that a physician should be consulted before making any healthcare decisions.
Saving one million hours by using AI to create 50,000 plain language summaries
“Three factors are converging in 2023 – the rise of public-facing generative AI technology, a resurgence in transparency initiatives with global health authorities, and mounting concerns related to health literacy despite major technological and scientific advances in recent decades,” said Zach Weingarden, Director of Product Solutions, TrialAssure.
Weingarden continues, “Plain language summary documents are notoriously time-consuming and can be challenging to write, and many pharmaceutical companies struggle to juggle resources between this and writing the required clinical study reports, completing other disclosure requirements, and filing their new drug applications. AI has the potential to turbocharge the initial drafting of these documents, automatically and in bulk, saving the skilled reviewers’ effort for a quick confirmation and finalization step. We have demonstrated this is possible by creating fully written drafts based solely upon data already available in public records.”
When manually drafting a plain language summary in the pharmaceutical industry, it takes approximately 20 hours per document to write from scratch. If this estimate is accurate, to draft 50,000 plain language summary documents would take approximately 1,000,000 hours. TrialAssure was able to reduce this workload to less than one day. Time, effort and cost no longer remain an excuse for transparency.
Accuracy of AI-generated plain language summary documents
“We hope this exercise will show that these AI-generated drafts are a great start, but AI has limited abilities and the use of AI is not a complete replacement of a skilled human writer,” said Weingarden. “Furthermore, we are limited to the data that is available in the ClinicalTrials.gov record. Depending on the structure and format of the data, it may be difficult for the AI to interpret and summarize accurately. We expect this to be an insightful demonstration of a committed path forward towards transparency, harvesting the scalable power and working to improve the limitations of our AI technology and welcome feedback from the scientific community.”
Feedback on the plain language summaries posted to TrialResults.com and generated by TrialAssure’s LINK application can be emailed to connect@trialassure.com and will help improve the quality of the plain language summaries.
With industry feedback incorporated and further Sponsor collaboration, TrialAssure will look to implement a new process in the near future that will use AI to automatically generate a plain language summary whenever a new record is available. This will scan ClinicalTrials.gov data daily, allowing new plain language summary documents to be available on TrialResults.com in near real-time.
Search the database at www.trialresults.com.
About TrialAssure
TrialAssure, Inc. is a leading, global clinical trial disclosure and data transparency provider with unmatched experience in helping navigate complex regulatory compliance challenges. TrialAssure provides fast, affordable, and intelligent software and service solutions to facilitate clinical trial disclosure (registration and results reporting), document and data anonymization, and enhanced patient engagement, while delivering the highest quality clinical trial transparency deliverables that exceed the most stringent requirements. Established in 2009, TrialAssure regularly adapts to ever-changing clinical trial disclosure and data transparency requirements and was named Data Solution of the Year – Healthcare in the Data Breakthrough Awards.
For more information, visit www.trialassure.com
